ILA to fast track approvals with the FDA? We will find out by the 12th of November
Our biotech Investment Island Pharmaceuticals (ASX: ILA) will find out in ~ two months whether or not its Marburg treatment into a $200 - $500M cash generator within the next 12 months…
Yesterday, ILA submitted a package of documents to the FDA including pharmacokinetic, safety and animal study results, as well as supporting documentation around use of Animal Rule…
ILA expects to hear back from the FDA on or before the 12th of November (US time).
That’s when we will know if ILA’s drug (Galidesivir) is eligible for fast tracked approvals…
IF the FDA gives the company the greenlight to pursue fast tracked approvals, ILA will start animal studies for Marburg Disease chasing:
- A priority review voucher (PRV) that comes in if ILA’s Galidesivir is FDA approved) - these can be worth on average US$150M.
- US Government National Stockpiling deals - these can be worth anything between US$100M to US$1.2BN ANNUALLY (More on this later).

(Source)
ILA going for fast tracked approvals using the “animal rule” process
ILA’s Marburg drug (Galidesivir) has already had US$70M spent on development (most of which was funded by the US government).
Marburg disease is classified as a Category A bioterrorism threat with a fatality rate of up to 88% in humans - Category A is the highest level threat by the US government. (source)
(for Category A bioterrorism threats, governments around the world will generally maintain a stockpile of vaccines or treatments to quickly deploy).
There is no current vaccine or cure for Marburg (and no strategic stockpile).
Which explains why the US would have spent US$70M on Galidesivir…
The drug has been tested across ~20 viruses in clinical trials before and is proven to be safe in humans already.
ILA’s strategy to skip the usual ~10+ year FDA approval process and monetise the drug in under 12 months is to apply for approvals under a little known rule that can fast track drugs to market.
That process is called FDA’s “ Animal Rule”.
The Animal Rule allows for drugs to be fast-tracked to market because of how deadly these conditions can be (and the urgency around defending against bioterrorism and bioweapon threats).
ILA’s drug has shown 100% survival rates for animals 24-48 hours after infection relative to placebo where survival rates are 0%.
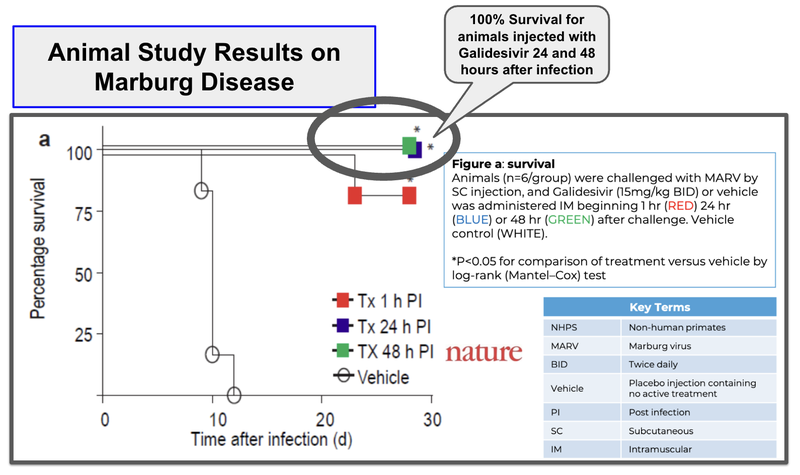
(Source)
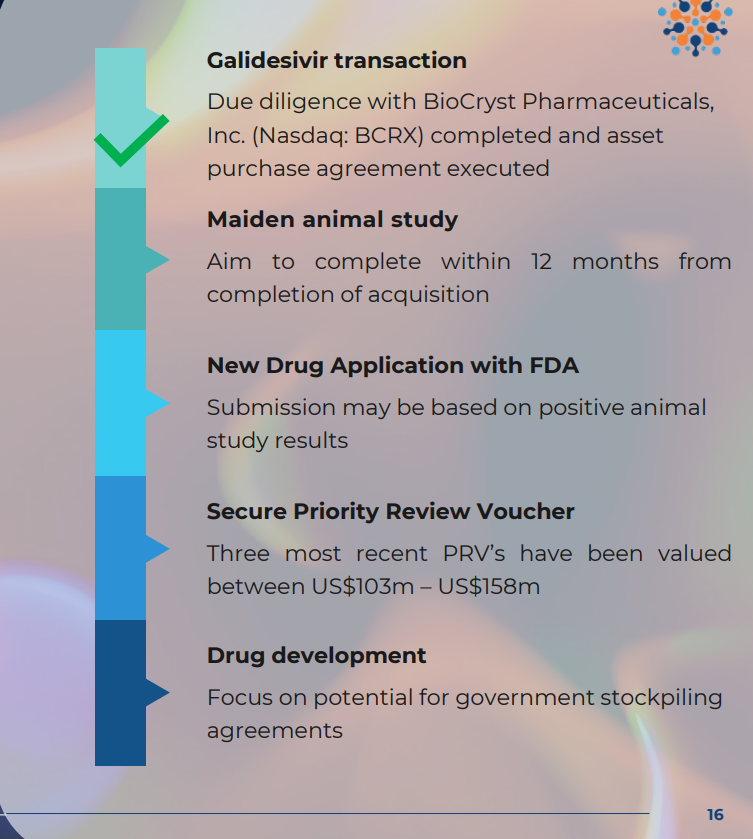
IF ILA is given the green light for approvals via the animal rule:
- THEN ILA will conduct a short, low-cost clinical trial on non-human primates (trial expected to cost circa $4M. ILA held $7.25M cash at June 30 so is funded for this) to evaluate if its drug is effective at treating Marburg disease.
UPDATE today: “Negotiations with potential strategic counterparties to advance planned animal study in Marburg reaching final stages” and ILA expects to complete the study in Q4 of this year (which starts on Wednesday).
- THEN, ILA will submit to the FDA for an IND approval of its drug (6 month review timeframe)
- IF APPROVED…
- SECURE a Priority Review Voucher, which is a tradeable asset worth on average ~US$150M (think of this like a ‘thank you’ for developing the drug - more on this later)
- SECURE commercial stockpiling contracts with the US Department of Defence for protection against bioweapons. These contracts can be very lucrative.
(Source)
In the short term though, the major catalyst will be what comes back from the FDA on or before the 12th of November.
ILA’s CEO David Forster and Chair Jason Carroll ran through a really solid presentation a few weeks back - this slide on the stockpiling deals (ranging between US$100M and US$1.2BN) was particularly interesting.
Check out the full webinar here

What’s next for ILA?
Animal trial for Marburg Disease
Now that ILA has completed the acquisition of Galidesivir, we want to see the company work with the FDA to develop an animal trial to determine efficacy on Marburg Disease.
🔄 FDA meetings to determine the application of the Animal Rule.
🔲 Clinical trial design completed
🔲 Clinical trial starts
🔲 Clinical trial completed
🔲 Clinical trial results
We think this should be a fairly quick process assuming ILA gets a favourable FDA Animal Rule outcome and it doesn't need to run trials in humans.

(Source)